Lipid Composition Effect on Liposome Properties: Role of Cholesterol in Cannabinoid Formulations
December 2020 | By Dr. Natalia Sannikova, Research & Development Manager at Ascension Sciences
Originally Published in PharmaExcipients
Introduction
Solubility of hydrophobic drugs such as cannabinoids can be greatly improved by liposome encapsulation. Lipid composition can be adjusted depending on the type of drug molecule or target tissue. Specific lipid excipients can alter liposome properties such as drug retention, size, and surface charge, and significantly influence liposome efficacy as nanomedicines.
Cholesterol is an integral part of biological membranes and plays an important role in the physical properties of liposomes [1]. Amount of cholesterol in the formulation affects the size of liposomes, encapsulation efficiency (EE%) and stability over time. Additionally, cholesterol is known to influence drug release profiles in liposomal formulations [2].
To examine the effect of cholesterol on the THC-loaded liposome properties, the cholesterol content was systematically varied in a series of POPC/cholesterol/DSPE-PEG2000 formulations prepared on the NanoAssemblr Benchtop instrument. The NanoAssemblr platform has previously been used for the preparation of unilamellar liposomes composed of synthetic phospholipids and loaded with hydrophobic drug molecules [3, 4].
Materials and Methods
POPC and DSPE-PEG were purchased from Avanti® Polar Lipids, cholesterol was purchased from Sigma Aldrich.
POPC, cholesterol and PEGylated lipid were dissolved in absolute ethanol. Calcium- (Ca2+) and magnesium- (Mg2+) free PBS buffer at pH=7.4 was used as the aqueous phase. The organic and aqueous phases were rapidly mixed using the NanoAssemblr Benchtop microfluidic instrument to form unilamellar liposomes of various sizes. THC was loaded in situ by dissolving it in the lipid solution. Formulations were then dialyzed against PBS for ethanol removal. Formulations were diluted down to 10% ethanol immediately following the mixing process and before dialysis, since high amounts of ethanol can destabilize liposomes.
Particle size and integrity were measured before and after dialysis using Dynamic Light Scattering (Zetasizer, Malvern Instruments, UK). Size and polydispersity index (PDI) were represented as the mean of 3 samples, error bars representing standard deviation of the mean. Cannabinoid content was quantified by HPLC.
| Lipid composition | POPC : Chol : DSPE-PEG |
| Organic solvent | Ethanol |
| Aqueous solvent | PBS pH 7.4 |
| Solvent Removal | Dialysis |
Results and Discussion
Liposomes with varied cholesterol content have been prepared with identical instrument parameters and API loads. As shown in Fig. 1, addition of cholesterol leads to a liposome size increase from 30.1 ± 0.4 nm to 51.6 ± 0.1 nm.
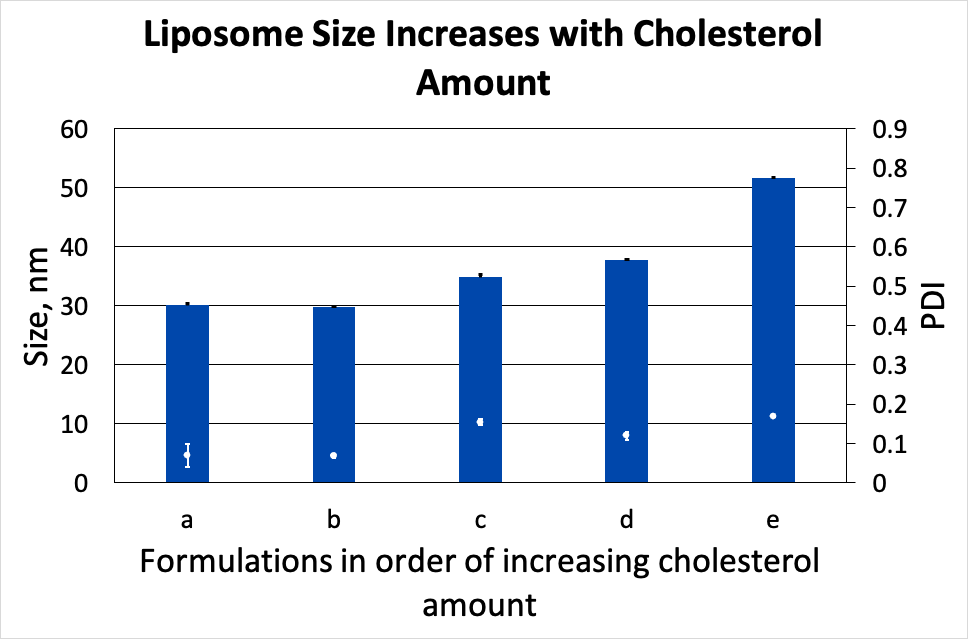
Figure 1. Liposome size increases with cholesterol amount.
We have observed that ethanol removal by dialysis resulted in liposome size increase. However, all prepared formulations demonstrated reasonable stability upon storage at 4°C. Liposomes with the highest cholesterol content are the most stable with size and PDI not changing significantly during 1 month of storage (Fig. 2).
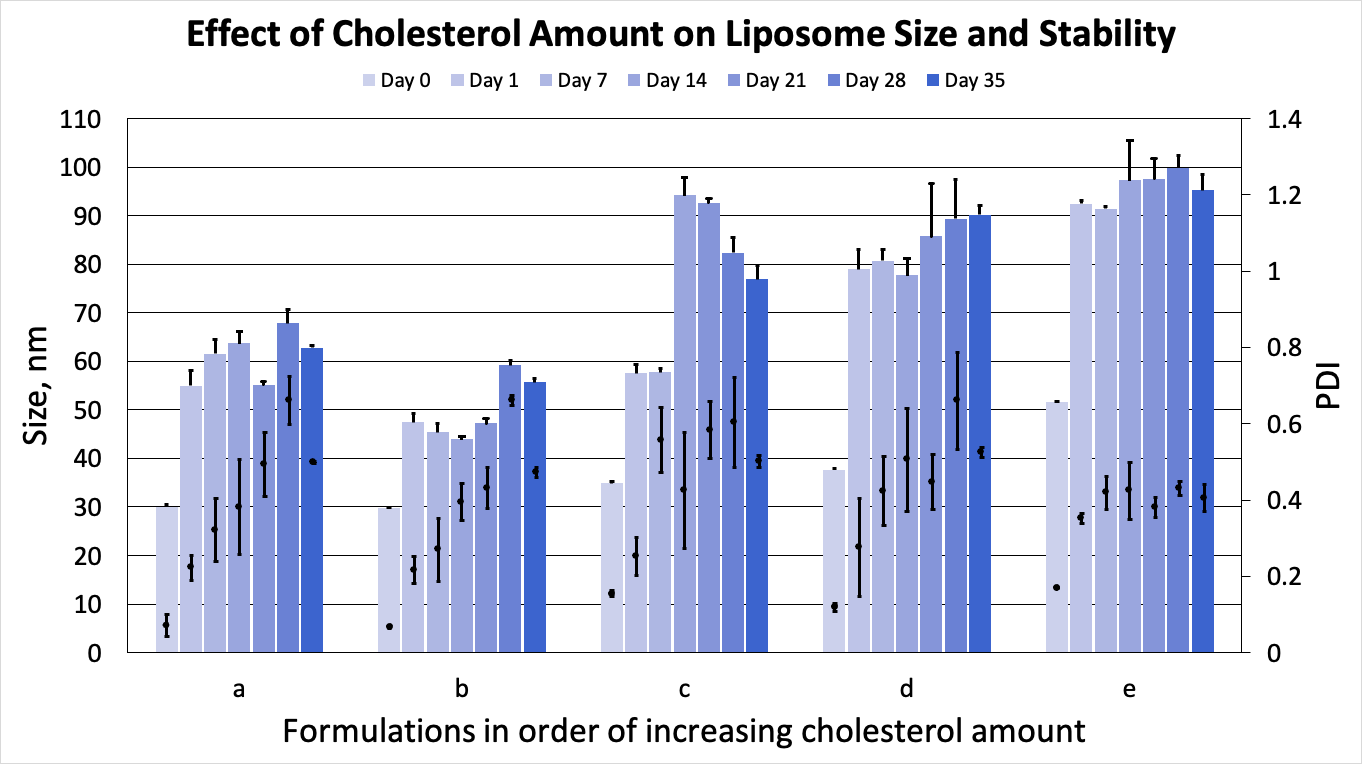
Figure 2. Effect of cholesterol amount on liposome size and stability.
From Figures 1 & 2, it is clear that liposome size increases with the proportion of cholesterol in the formulation. This can be explained by considering the effect of cholesterol on the lipid bilayer. It has been demonstrated that the mechanical rigidity of the lipid bilayer consisting of phospholipids with at least one saturated tail, for example, POPC as in our study, increases in presence of cholesterol [1]. This rigidity limits the curvature of a bilayer, leading to larger equilibrium liposome size.
Liposome encapsulation efficiency (EE%) has been measured post formulation. Cholesterol-rich liposomes have shown slightly lower EE% compared to the formulations prepared with lower amounts of cholesterol: 72% vs 85 – 88% (shown in Fig. 3 in darker blue colour).
We then measured THC content in the liposomes after one month of storage at 4 °C. Results are shown in Fig. 3 (light blue bars). Increase of cholesterol in lipid mixture leads to better drug retention characteristics.
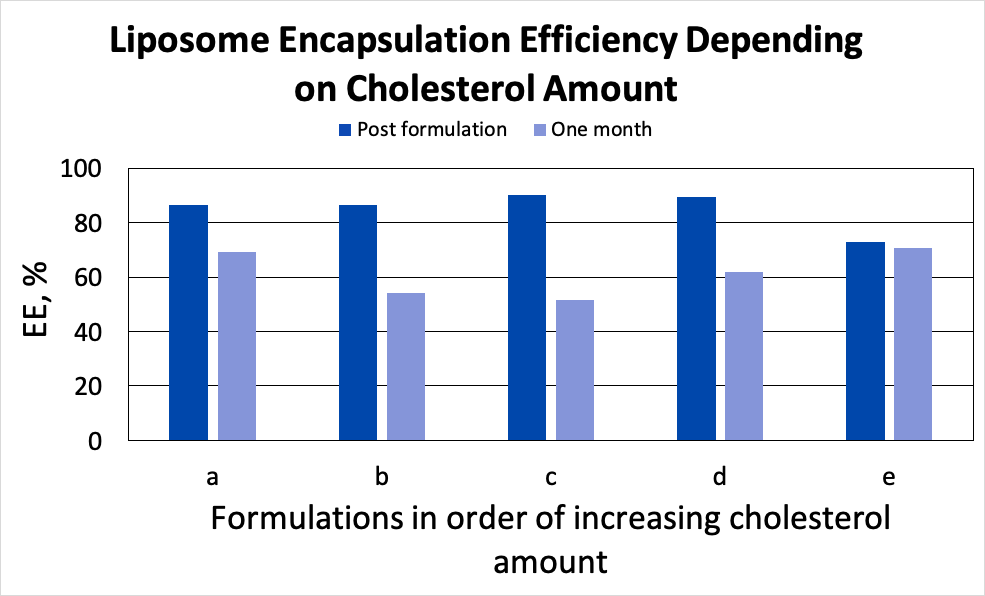
Figure 3. Liposome encapsulation efficiency depending on the cholesterol amount.
Cannabinoids and terpenes of interest are structurally similar to cholesterol and can potentially interact with phospholipids in the same manner. This can explain lower EE% in cholesterol-rich liposomes: both cholesterol and THC are incorporated into the lipid bilayer and higher amounts of cholesterol limit the space available for API encapsulation [2]. Increased drug retention ability of the same formulation can be also traced back to the competitive interaction between API and cholesterol [5].
Conclusion
Formulation parameters such as lipid composition can be used to tune liposomes size, stability and encapsulation efficiency. The amount of cholesterol in a liposome formulation significantly impacts the size of vesicles. In case of THC-loaded POPC/Chol/DSPE-PEG formulations, a greater proportion of cholesterol favors the formation of larger liposomes with lower encapsulation efficiency and increased drug retention.
References
- Pan, J. et al. Cholesterol Perturbs Lipid Bilayers Nonuniversally. Physical Review Letters 100, (2008).
- Briuglia, M.-L. et al. Influence of cholesterol on liposome stability and on in vitro drug release. Drug Delivery and Translational Research 5, 231–242 (2015).
- Zhigaltsev, I. V. et al. Bottom-Up Design and Synthesis of Limit Size Lipid Nanoparticle Systems with Aqueous and Triglyceride Cores Using Millisecond Microfluidic Mixing. Langmuir 28, 3633–3640 (2012).
- Zhigaltsev, I. V.et al. Production of limit size nanoliposomal systems with potential utility as ultra-small drug delivery agents. Journal of Liposome Research, 1–7 (2015).
- Mohammed, A. et al. Liposome formulation of poorly water soluble drugs: optimisation of drug loading and ESEM analysis of stability. International Journal of Pharmaceutics 285, 23–34 (2004).
About ASI
Employing nanoparticle formulation technology from the cutting edge of genetic medicine, Ascension Sciences is developing cannabinoid nano delivery platforms and techniques for the pharma and nutraceutical industries. Liposomes, nanoemulsions, lipid nanoparticles and polymeric nanoparticles have all shown promise in improving the therapeutic benefits of cannabinoids, but the full potential of these therapies has not yet been unlocked.
Our R&D and formulation development services are an efficient option for research driven firms that require the advantages of nanoparticle delivery for their active ingredients. We work with other passionate researchers in the industry that share a common goal of improving the human condition and bringing novel therapies to those who need them most.
For more information, please visit: https://ascensionsciences.com/
